The history of soap manufacturing
Soaps can be
prepared through saponification. What is saponification?
therefore,
general equation,
What is fat?
What happen to the fat during saponification?
Consider this reaction;
hydrolysis reaction
Fats/oil in
concentrated sodium hydroxide à Fatty acid
+ glycerol
Fatty acid + alkali/sodium ion à sodium salt fatty acid/soap
+ water
Overall: Fats/oil
+ alkali/sodium ion à soap
+ glycerol
Preparation of soap by saponification
Procedure :
1.
10 cm3
of palm oil is measured with measuring cylinder 10ml and poured into a beaker
250ml.
2.
50 cm3
of concentrated sodium hydroxide solution 5 mol dm-3 measured with measuring
cylinder 50ml and poured in the beaker.
3.
The
mixture is heated and stirred with glass rod until its boiling for 5 minutes.
Then, the flame is turned off and the mixture is left to cool.
4.
50 cm3
water and a few spatulas of sodium chloride was added to the mixture and
boiled again for 5 minutes. Then, the flame is turned off and the mixture is
left to cool.
[white
precipitate is formed and floats].
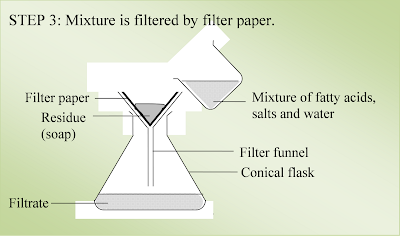
5.
The mixture
is filtered with filter paper, and the residue (soap) is washed by distilled
water.
Example-->
Soap Chemical
formula
Sodium palmitate CH3(CH2)14COONa
Sodium stearate CH3(CH2)16COONa
Sodium oleat CH3(CH2)CH=CH(CH2)COOK
- Potassium soap is usually used for bathing because its more softer and milder than sodium.
BARAKALLAHUFIKUM










setai mehh....naah ! aq bg adiah :D
ReplyDeletengeee!
Deletetrimas ya cik iwana !